Electrochemistry is pervasive throughout many aspects of our lives — from metabolic redox reactions that sustain our bodies, to biochemical sensors for healthcare monitoring, batteries for personal electronics and electric vehicles, and industrial productions of chemicals and materials.
The profound technological and industrial impacts of electrochemical processes derive from their scalability and our ability to control the electrochemical reactions with a simple electrochemical-potential knob (i.e., the applied potential). In the face of growing concerns about anthropogenic CO2 emissions and related climate change, scientists and engineers are looking to electrochemistry as an approach to address the global challenge of rising atmospheric CO2 levels. Rileigh, Kelsey and Leila recently created a great comic to illustrate the underlying challenges and our scientific and technological endeavors towards a sustainable solution; check out the comic here (COMIC).
We are intrigued by the prospects of developing electrochemical routes to valorize CO2. Specifically, to utilize emerging renewable electricity inputs to transform CO2 from an environmental liability to a feedstock for carbon-based materials. A critical challenge of bringing this vision to fruition is to develop catalysts and electrochemical systems that can efficiently reduce CO2 to value-added molecules (e.g., ethylene) at industrially relevant conditions with appropriate durability, lifetime, and product selectivity.
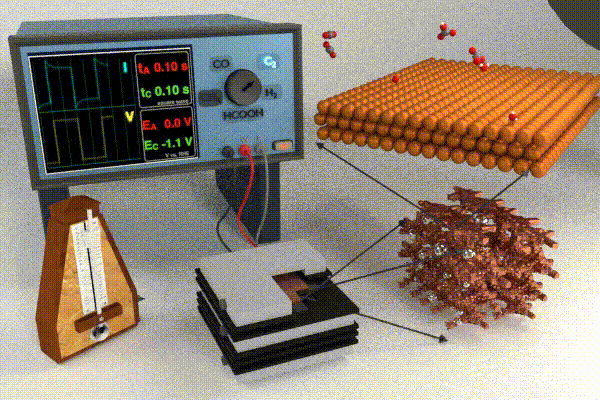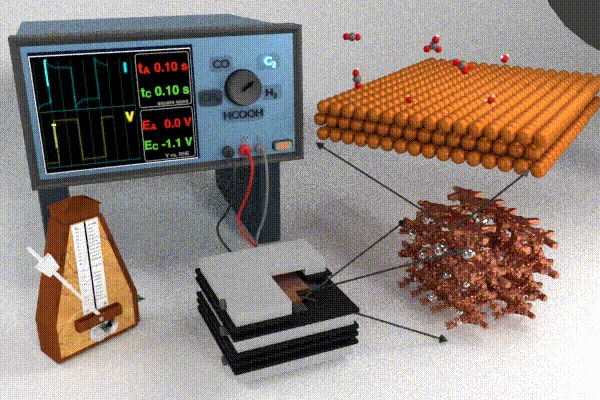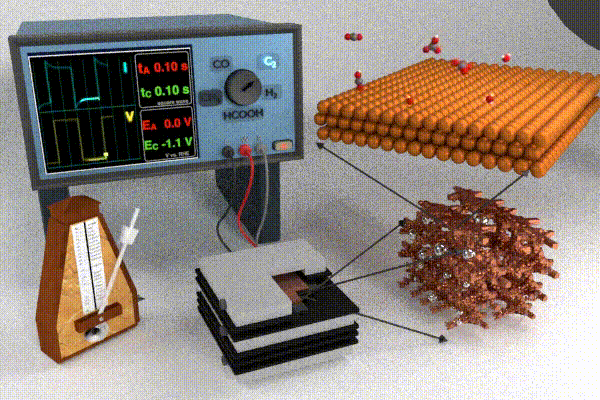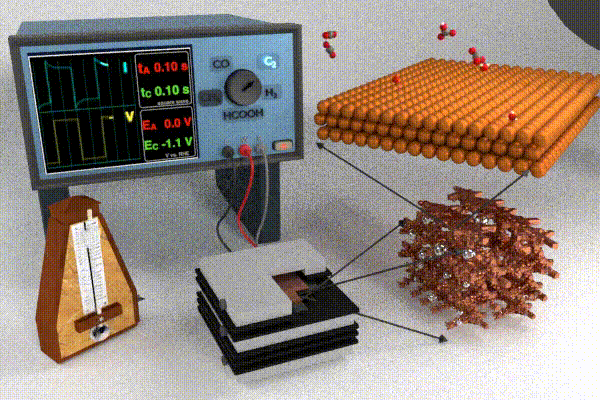
Research strategies to address these challenges have primarily focused on increasing the catalyst activity through changing the catalyst structure and composition (e.g., nanostructuring or alloying), modifying the electrolyte composition, or improving the electrolyzer cell design. Each of these design strategies attempts to improve and/or stabilize processes at the interface by modifying adsorbate coverage, improving reactant transport, enhancing surface roughening/restructuring, and causing surface oxidation. Our scientific endeavors in this field have pursued a slightly different approach, which is to dynamically modulate the conditions at the electrode / electrolyte interface by applying the potential in a pulsed form. Varying the temporal profile of the applied potential pulse impacts the local electrochemical environment (i.e., the double layer) near the catalyst surface which has been shown to affect the transport and reaction processes listed above. Pulsed electrochemical CO2 reduction (p-eCO2R) has garnered interest by virtue of providing a low cost and responsive in situ modification to modulate product selectivity, stability, and activity. Whereas a pulsed potential is simple to apply, our current understanding of the underlying mechanisms by which the pulse impacts underlying physicochemical processes in the dynamic microenvironment near the catalyst surface is still at a relatively early stage. For more information on the anticipated opportunities in p-eCO2R check out our recent perspective paper.
Our approach to advancing fundamental understanding of pulsed electrochemical CO2 reduction focuses on understanding the impact of dynamic changes in surface adsorbates, electric double layer and transport. This work involves electrochemical experiments, spectroscopy and computational simulation. Our overarching objective is to establish the scientific and engineering foundation to design electrochemical processes that enable unprecedented control over product selectivity tuned by the optimization of the potential pulse profile. Building on emerging fundamental insights into the behavior of the electrode/electrolyte interface subjected to a pulsed potential from we are now pushing forward to investigate how these effects play out in porous electrode structures and other electrolyte combinations.
We see compelling synergies between concurrent projects focused on p-eCO2R and additive manufacturing of mesoporous hierarchical structures. Gas-diffusion electrodes in particular stand to benefit from advanced manufacturing capabilities by virtue of the fact that optimizing their structure requires consideration of gas-, liquid-, and charge-transport spanning multiple time and length scales. The ability to design hierarchical porous gas diffusion electrodes and to test their performance in pulsed electrochemical reactions presents an exciting opportunity to significantly advance the concept of creating ‘electronic leaves’.

