Congratulations to Rileigh, Kevin, Kelsey, Jessica, Jiyoon, and Tyler for publishing the paper “Effect of Electrolyte Composition and Concentration on Pulsed Potential Electrochemical CO2 Reduction” in ChemElecroChem (see the full paper here link).
Electrochemical CO2 reduction (eCO2R) on copper has garnered strong interest as a promising pathway to convert CO2 emissions into higher value chemicals including fuels and hydrocarbon feedstocks. We previously reported (Kimura et al. ChemSusChem (2018)) that the application of a pulsed potential during eCO2R improves selectivity toward converting CO2 to higher order products, suppresses undesirable H2 production, and maintains electrode stability. The working hypothesis which emerged from the initial study was that the pulsing-dependent product selectivity was related to dynamic changes in species adsorbed to the electrode surface. Subsequent studies have pointed to interesting dynamic processes at the solid-electrolyte interface (SEI). In the specific context of electrochemical CO2 reduction, the composition and concentration of the electrolyte are known to impact product selectivity. How the dynamic processes and electrolyte effects at the SEI play out in the case of pulsed potentials has not yet been investigated. Pulsed potential experiments thus present an intriguing opportunity to establish deeper insights into these processes.
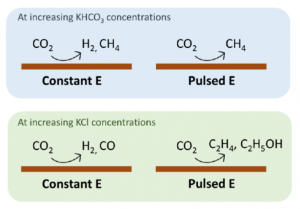
We found that the relationship between electrolyte concentration/composition and product distribution for pulsed potential eCO2R is different from constant potential eCO2R. In the case of constant potential eCO2R, increasing KHCO3 concentration is known to favor the formation of H2 and CH4. In contrast, for pulsed potential eCO2R, H2 formation is suppressed due to the periodic desorption of surface protons, while CH4 is still favored. In the case of KCl, increasing the concentration during constant potential eCO2R does not affect product distribution, mainly producing H2 and CO. However, increasing KCl concentration during pulsed potential eCO2R persistently suppresses H2 formation and greatly favors C2 products, reaching 71% Faradaic efficiency. Collectively, these results provide new mechanistic insights into the pulsed eCO2R mechanism within the context of proton-donator ability and ionic conductivity.